Tox By Design is proud to announce a recent partnership with Avicenna Alliance Association, a key player in the promotion of in-silico methodologies, as of 2024. As a participating member, Tox By Design is involved in the education of stakeholders on the subject of in-silico usage and global regulation. Alongside our industry partners, we campaign for a globally harmonized regulatory framework on in-silico methods that decreases uncertainty and streamlines relevant reporting processes, such as the (Q)SAR Assessment Framework provided by the EMA.
How do AI in-silico (Q)SAR Computational Modeling & Simulation methods benefit the pharma-biotech ecosystem?
- Reduced drug development costs via early lead candidate optimization and reduced time-to-market for R&D
- Improved patient safety via improved cross contamination containment measures on multiple purpose biotech shared production lines at commercial stage
- More robust data collection derived from preclinical tox animal studies to aid in negotiating design of clinical human trials I and IIa with regulatory bodies
- Minimized animal testing due to use of in-silico NAMs (New Alternative Methodologies) abiding by three R’s regulation
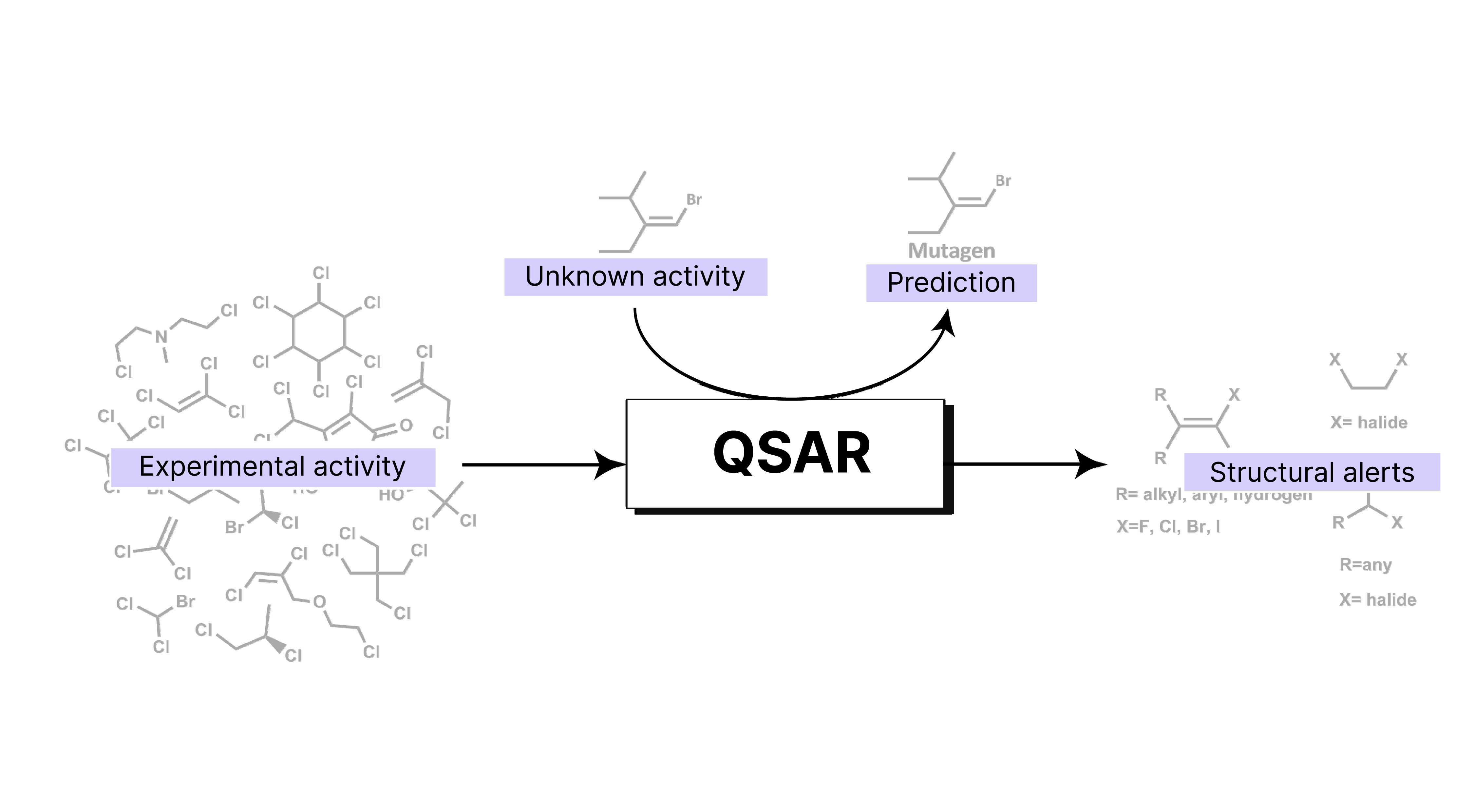
How is toxicological endpoint calculation streamlined due to innovation in AI in-silico (Q)SAR CM&S methods?
Tox by Design has been using Quantitative Structure-Activity Relationships (Q)SAR protocols as early as 2016. To leverage all its potential in the toxicological domain, Tox By Design has assembled an expertise of ERT board-certified toxicologists and PharmDs alongside a top PhD Organic chemist to interpret (Q)SAR data.
We recognize the brilliant future of in-silico predictive software for toxicological modeling. Computational modeling and simulation is already delivering within the drug development ecosystem, and nowhere is it exemplified more than by pharmaceutical toxicology.
For US based companies, please note that you can benefit from our Gold Supplier Partnership with Scientist.com, a streamlined, AI powered R&D procurement platform.
If your company is based in India, you can benefit from simplified tax filing and ensured regulatory compliance thanks to our PAN card registration.